The presence of the phenanthroline favors the binding of guests able to establish a hydrogen bond with the strap.1 In particular, N-unsubstituted imidazoles (hydrogen emphasized in Figure 2 left) are efficiently recognized and bound in the strap.2 This specific recognition has been use as a glue and iterated in self-assembly processes which have led to porphyrin wires observable on surfaces from hundreds of nanometers3 to µmeter long.4
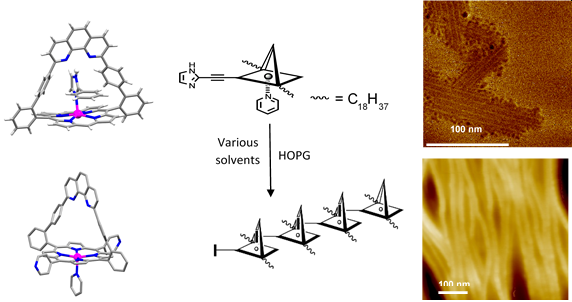
Fig. 2. X-ray structure showing the selective inclusion complex of 2-phenyl-imidazole inside the strap (top left), X-ray structure showing the coordination of pyridine outside the strap (bottom left), cartoon representation of the building block and the assembly on a surface (center), AFM images obtained from deposit on HOPG in methylcyclohexane (top right) and dichloromethane (bottom right).
Linear assemblies of porphyrins on surfaces can also be designed through peripheral interactions.5 A second long-time approach has been developed based on the design of external coordination sites on the edge of porphyrin macrocycles in which evidence for very strong electronic interactions between the porphyrins has been gathered by electro and photo-chemistry.6
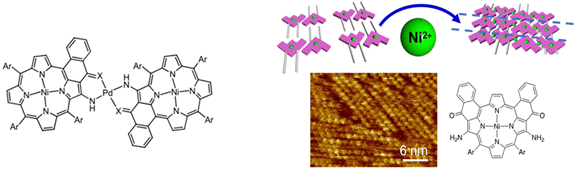
Fig. 3. Porphyrin dimer linked by a Pd coordination complex illustrating the principle of the assembly (left), in situ formation of Pd complexes on HOPG (right).
Extremely efficient and regular arrangement can be obtained by successive deposition of the ligand on HOPG followed by in situ formation of the external metal complex.7
- Side Selection of the Fifth Coordinate with a Single Strapped Zinc(II) Porphyrin Receptor: Binding Studies and X-ray Characterization of Two Imidazole Complexes J. Am. Chem. Soc. (1997), 119, 12362-12363
- Induced Fit Process in the Selective Distal Binding of Imidazoles in Zinc(II) Porphyrin Receptors Inorg. Chem. (2003), 42, 3779-3787.
- Highly Linear Self-Assembled Porphyrin Wires Inorg. Chem. (2011), 50, (13), 6073-6082; Surface-Tuned Assembly of Porphyrin Coordination Oligomers J. Am. Chem. Soc. (2008), 130, 9994-10001.
- Dynamic Assembly of Porphyrin Wires Trapped on a Highly Oriented Pyrolytic Graphite Surface Org. Lett. (2012), 14, (8), 1998-2001; Trapping Nanostructures on Surfaces through Weak Interactions Chem. Eur. J. (2015), 21, 13437-13444.
- Metal-mediated linear self-assembly of porphyrins Chem. Commun. (Feature Article) (2018), 54, 1550-1558.
- Syntheses and Optical and Electrochemical Properties of Porphyrin Dimers Linked by Metal Ions J. Am. Chem.Soc. (2002), 124, 6168-6179; Porphyrins acting as external and internal ligands: preparation of conjugated trimetallic dimeric porphyrins Chem. Commun. (2001), 91-92; “Metal Linkage Effect on Ultrafast Singlet Energy Transfer”, Chem. Eur. J. 2016, 22, 10484-10493.
- Synthesis and Study at a Solid/Liquid Interface of Porphyrin Dimers Linked by Metal Ions Inorg. Chem. (2017), 56, (24), 15081-15090; Coordination-Driven Construction of Porphyrin Nanoribbons at a Highly Oriented Pyrolytic Graphite (HOPG)/Liquid Interface J. Am. Chem. Soc. (2019), 141, 10137-10141.