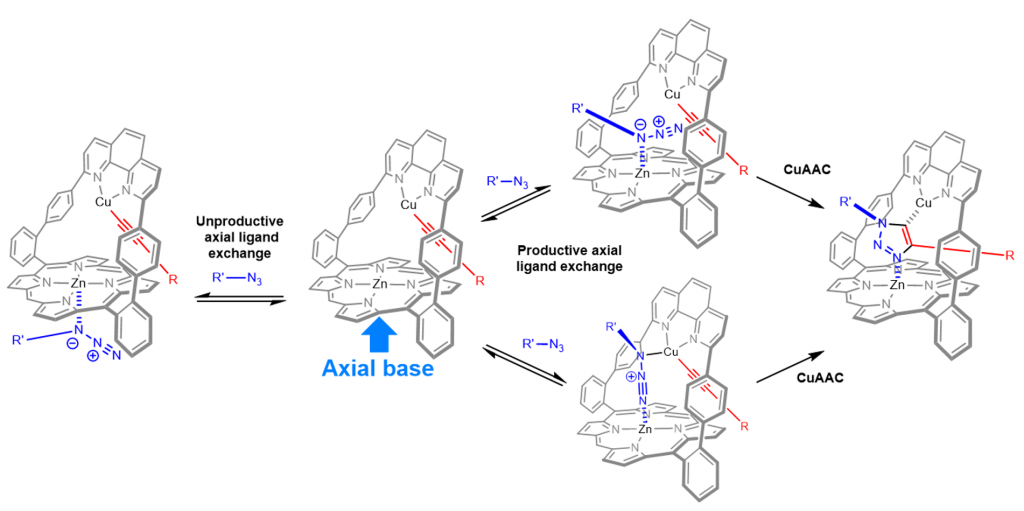
In the phenanthroline strapped porphyrin did not only provide a site for molecular recognition, it also provided a space for CuAAC reactions due to the presence of the phenanthroline binding site for copper (Figure 4). As a result, the use of bulky alkyne and azide stoppers led to the preparation of various rotaxanes species (X-ray structure Figure 4 right).1
 |
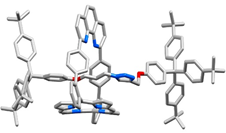 |
Fig. 4. Mechanism proposed for the tandem activity of zinc and copper in CuAAC (left), X-ray structure of a rotaxane containing a free-base porphyrin in the macrocycle (right).
Using this approach, a fullerene stoppered rotaxane has been prepared. Time resolved fluorescence studies performed in the group of Prof. D. Guldi in Erlangen have shown that excitation of the porphyrin induces a motion of the fullerene stoppered dumbbell and leads to long lived charge separated species in coordinating solvents. 2