In the past two decade, many electroactive structures developed in the group used porphyrin as their redox addressable sub-unit. For example, the co-facial bis-porphyrin(Ni) species (PacMan bis-porphyrins) shown in Figure 5 could be opened and closed by electrochemistry due to the use of a crown-ether derivative of calix[4]arene as a spacer.1 Synthetic difficulties and the opportunity for a nice collaboration with C. Bucher prompted synthetic efforts targeting scaffolds in which the porphyrin is replaced by a viologen unit.2
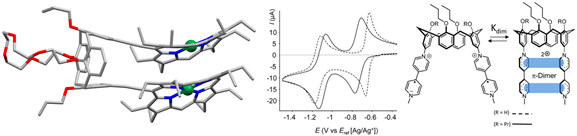
Fig. 5. An electro-addressable PacMan bis-Ni porphyrin (left), and an electrochemically switchable scaffold built with viologen derivatives (right).
More recently, switchable structures, such as the series of cyclophanes in Figure 6, that can be redox triggered have been designed using a viologen subunit as the electroactive part.3
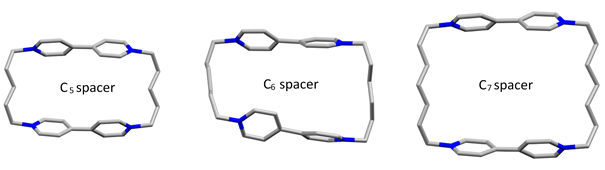
Fig. 6. X-ray structures of flex-boxes (flexible blue-boxes) three cyclophanes of viologen that show different flexibilities and different rates of π-dimer formation in spectroelectrochemistry.4
Engaging in the field of viologens has also been the opportunity to develop or revisit the synthesis of several bipyridines. Among these, the preparation of a previously reported viologen tetraester derivative has been improved. EPR and electrochemical studies have shown that the compound in Figure 7 forms extremely stable radical species.5

Fig. 7. Structure of a viologen tetraester derivative 12+ (left) and 1+. stability vs MV+. in various conditions(right).
- Electrochemically Triggered Open and Closed Pacman Bis-metalloporphyrins J. Am. Chem. Soc. (2006), 128, 3488-3489; Unsymmetrical Calix[4]arene Bisporphyrin Pacman
Org. Lett. (2007), 9, 785-788; “Evidence for Dual Pathway in Through-Space Singlet Energy Transfers inFlexible Cofacial Bisporphyrin Dyads”, Chem. Eur. J., 2009, 15, 524-535. - Hydrogen-Bond Controlled π-Dimerization in Viologen-Appended Calixarenes: Revealing a Subtle Balance of Weak Interactions Org. Lett. (2015), 17, 4058-4061.
- Viologen cyclophanes: redox controlled host-guest interactions Chem. Comm. (2015), 51, 15772-15775.
- Flexible Viologen Cyclophanes: Odd/Even Effects on Intramolecular Interactions ChemPhysChem (2017), 18, 796-803; Pairing-up viologen cations and dications: a microscopic investigation of van der Waals interactions Phys. Chem. Chem. Phys. (2018), 20, 27878-27884.
- A Highly Stable Organic Radical Cation Org. Lett. (2018), 20, 8004-8008.