We are interested in the complexation of substrates of environmental, biological, and toxicological interest in order to optimize their detection using very sensitive methods including luminescence and mass spectrometry. To this aim, we have been working on the design and the synthesis of receptors for neutral molecules and ions, and ligands for metal cations. Molecular architecture plays a central role in our scientific reasoning. Indeed, the design of these systems uses the concept of preorganization in order to maximize the control of the formation of the desired structures, and to optimize the stability of the resulting complexes.
More specifically, we have developed:
– Molecules featuring a hydrophobic cavity for the encapsulation of non-polar substrates[1] or cations via p electron-cation interactions[2];
– Cage-like ligands for the encapsulation of specific cations (H+, Cu+), and the study of the effect of encapsulation on the molecular structure of the receptor (e.g., dissymmetrization[3]) or, reciprocally, the effect of the structure of the ligand on the coordination behavior of the encapsulated cation (e.g., unusual coordination environment/geometry, unusual metal complex dynamics[4]).
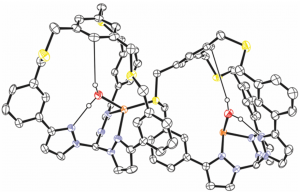
Fig. 7. ORTEP view of a coordination polymer involving a Cu+-complexed tris-pyrazolylmethane-based molecular cage in which a water molecule is bound to the receptor and to Cu+ (ellipsoid in orange).
Recently, we have focussed our research in two directions:
– The metallo-organic cryptophanes, a class of molecular cages that belong to the family of receptors invented by the late André Collet, which, unlike the fully organic original systems, incorporate transition metal cations as assembling units. Our approach uses square planar palladium and platinum complex fragments and cyclotribenzylene functionalized with nitrile binding subunits. Remarkably, the self-assembly process is diastereoselective, generating the chiral, D3-symmetric cryptophanes.[5]
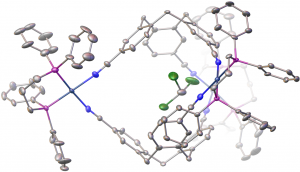
Fig. 8. ORTEP view of a pallado-organic cryptophane including a chloroform guest. The triflate anions are omitted for clarity.
– The sequestration of heavy M4+ metal cations. We synthesize bio-inspired ligands for the efficient complexation of highly toxic actinides such as Pu4+, and tetravalent transition metal cations, such as Zr4+, which finds applications in imaging by positron emission tomography, and represents a surrogate of Pu4+.[6] Our ligands use the hydroxamate function as metal binding fragment. This singly anionic oxygen-based chelate is found in natural siderophores, but has only scarcely been involved in synthetic coordination chemistry. This project is a joint venture with the group of Dr. Michel Meyer at the Institute of Molecular Chemistry of the Université de Bourgogne in Dijon (ICMUB).
References.
[1] C. Huo, J.-C. Chambron, M. Meyer, New J. Chem. 2008, 32, 1525-1536.
[2] (a) F. Brégier, E. Aubert, E. Espinosa, J.-C. Chambron, ChemistrySelect 2016, 1, 2389-2395; (b) A. Lélias-Vanderperre, E. Aubert, J.-C. Chambron, E. Espinosa, Eur. J. Org. Chem. 2010, 2701-2708; (b) F. Brégier, O. Hudeček, F. Chaux, M.-J. Penouilh, J.-C. Chambron, P. Lhoták, E. Aubert, E. Espinosa, Eur. J. Org. Chem. 2017, 3795-3811.
[3] C. Bonnot, J.-C. Chambron, E. Espinosa, K. Bernauer, U. Scholten, R. Graff, J. Org. Chem. 2008, 73, 7881-7881.
[4] L. Wang, J.-C. Chambron, E. Espinosa, New J. Chem. 2009, 33, 327-336.
[5] A. Schaly, Y. Rousselin, J.-C. Chambron, E. Aubert, E. Espinosa, Eur. J. Inorg. Chem. 2016, 832-843.
[6] (a) P. Jewula, J.-C. Chambron, M.-J. Penouilh, Y. Rousselin, M. Meyer, RSC Adv. 2014, 4, 22743-22754; (b) P. Jewula, J.-C. Berthet, J.-C. Chambron, Y. Rousselin, P. Thuéry, M. Meyer, Eur. J. Inorg. Chem. 2015, 1529-1541.